
Takin Ghavimi
University of Tehran, Iran
Title: Fabrication of new LSCF infiltrated BSCF material to increase electrochemical performance of fuel cells
Biography
Biography: Takin Ghavimi
Abstract
Introduction
A solid oxide fuel cell (SOFC) is an electrochemical conversion device that produces electricity directly from fuels. These fuel cells are comprised of ceramic electrolytes that can provide high efficiency in performance, fuel flexibility and overall low cost of the system [1]. The main disadvantage of these systems is the high operation temperature, which can result in (1) formation of an insulating layer, caused by reaction between electrode and electrolyte materials, (2) the necessity of using high cost interconnecting materials such as LsCrO3 and (3) possibility of crack formation caused by thermal coefficient mismatch between the electrode and electrolyte materials [2]. As is shown in literature, material characteristics in general can be modified using different thermal and environmental treatments and before and after fabrication and synthesis [3-10]. In our work, we investigate modification methods to reduce the operating temperature of SOFCs without sacrificing the system performance through fabrication of a new solution infiltrated Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF) cathode, employing the two most effective cathode materials BSCF (Ba0.5Sr0.5Co0.8Fe0.2O3-δ) and LSCF (La0.6Sr0.4Co0.2Fe0.8O3-δ).
Methods and materials
Ceramics were synthesized using co-precipitation method at precipitation pH of at least 8 and a calcination temperature of 1000°C. The structure of synthesized ceramics is studied using X-ray diffraction (Philips PW-1730).
Results and discussion
BSCF has the advantage of high ionic conductivity due to its high concentration of oxygen vacancies, enabling it to easily permit the diffusion of oxygen ions while LSCF is known to possess significantly higher electronic conductivity as well as better performance at operating temperatures of bellow 750 ºC [11,12]. We aim to achieve both characteristics of the individual components. To produce the infiltrated BSCF, we first used our developed method of co-precipitation synthesis, which was followed by structure analysis using X-ray Diffraction (XRD). The synthesized powders were then used to fabricate electrochemical half cells. These cells were infiltrated with a nitrate solution of LSCF and calcined at 1000 ºC for 5 hours to permit the LSCF to crystalize. The electrochemical performance of the half cells was then evaluated. Our results show the cells with infiltrated BSCF electrode possess higher electronic conductivity than those with pure BSCF electrodes.
Image:
A)
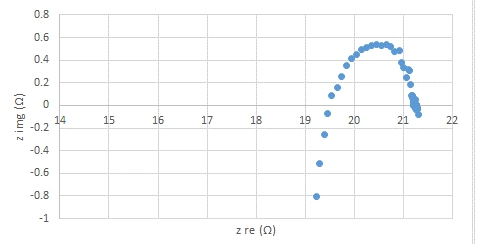
B)
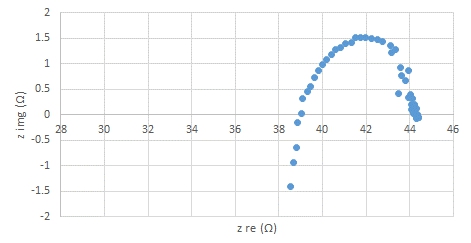
Fig.1. Impedcance spectra of (A) BSCF infiltrated LSCF and (B) LSCF at 600 ºC shows lower resistance and therofore higher electonic conductivity in BSCF/LSCF
Conclusion
The results of this investigation can be used in fabrication of fuel cells capable of operating in lower temperatures, which can reduce the overall costs of obtaining electricity and increase the performance efficiency.
References:
- Fergus J, Hui R, Li X, Wilkinson DP, Zhang J. Solid Oxide Fuel Cells: Materials Properties and Performance. CRC Press; 2008. 298 p.
- Singhal SC, Kendall K. High-temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications. Elsevier; 2003. 406 p.
- Chang J-K, Chen Y-L, Tsai W-T. Effect of heat treatment on material characteristics and pseudo-capacitive properties of manganese oxide prepared by anodic deposition. J Power Sources. 2004;135(1-2):344–53.
4. Najdahmadi A, Zarei-Hanzaki A, Farghadani E. Mechanical properties enhancement in Ti–29Nb–13Ta–4.6Zr alloy via heat treatment with no detrimental effect on its biocompatibility. Mater Des. 2014;54:786–91.
5.Kokubo T, Miyaji F, Kim H-M, Nakamura T. Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J Am Ceram Soc. 1996;79(4):1127–9.
6.Najdahmadi A, Zarei-Hanzaki A, Farghadani. Microstructural evaluation and mechanical properties of solution treated biomedical TNTZ alloy. International conference of metallurgical engineering society and foundry men’s society 1622-IMES-CONGR-FULL [Internet]. 2012 Oct; Available from: http://dx.doi.org/10.13140/RG.2.2.21846.96325
7. KochmaÅ„ska A, Garbiak M. High-Temperature Diffusion Barrier for Ni-Cr Cast Steel. Diffus Defect Data Pt A. 2011;312-315:595–600.
8. Najdahmadi A, Lakey JR, Botvinick E. Diffusion coefficient of alginate microcapsules used in pancreatic islet transplantation, a method to cure type 1 diabetes. InNanoscale Imaging, Sensing, and Actuation for Biomedical Applications XV 2018 Feb 20 (Vol. 10506, p. 105061D). International Society for Optics and Photonics
9. Wei C, Srivastava D, Cho K. Thermal Expansion and Diffusion Coefficients of Carbon Nanotube-Polymer Composites. Nano Lett. 2002;2(6):647–50.
10. Kummerfeld G, Krishnan R, Najdahmadi A, Botvinick E, Lakey JRT. Alginate composition and temperature influence microcapsule permeability. Royan international twin congress 11th congress on stem cell biology and technology. 2015/9;17(1):19–20.
11.Li S, Zhe L, Huang X, Wei B, Su W. Thermal, electrical, and electrochemical properties of Lanthanum-doped Ba0.5Sr0.5 Co0.8Fe0.2O3–δ. J Phys Chem Solids. 2007;68(9):1707–12.
12. Tsipis EV, Kharton VV. Electrode materials and reaction mechanisms in solid oxide fuel cells: a brief review. J Solid State Electrochem. 2008;12(11):1367–91.
