
Nazrin Abdullayeva
TOBB University of Economics and Technology, Turkey
Title: Development of Disulfonated Poly (arylene ether sulfone) copolymer membranes for Li+ flow batteries
Biography
Biography: Nazrin Abdullayeva
Abstract
Redox flow batteries (RFBs) constituting a large space in the electrochemical systems used in energy storage purposes are best known for their long-lasting life services, simple manufacturing, low cost and safety properties. Li-flow batteries being a representative of aqueous RFBs has a potential of becoming one of the major used battery types for its suitable and higher voltages in contrast to other RFBs based on proton chemistry. In Li-flow batteries, membrane acts as the separating layer between two electrolyte compartments that prevents the crossover of the charged molecules. The suitable membrane selection is a crucial factor that strongly affects the efficiency of the device. As far as we know, NafionTM has been widely used in the flow battery systems, so far. However, there is no significant study performed in the field of membrane optimization for Li RFBs. Herein, for the very first time we introduce disulfonated poly (arylene ether sulfone) (BPSH) copolymers as an alternative membrane for Li RFBs. Sodium form of copolymer has successfully been converted into acid and lithium forms. Fundamental material characterizations such as FTIR), Raman, TGA, AFM, H+ and Li+ conductivities, cyclic voltammetry and performance evaluations have been widely studied to identify chemical, electrochemical and structural properties of the membranes. Additionally, the influence of basic membrane properties such as water uptake, proton conductivities and the ion exchange capacities on the Li ion transport has been investigated. It has been shown that a correlation exists between the conductivity of lithiated membranes and molarity of conducting solution. The highest lithium conductivity was measured as 39.07 Scm-1 at 1M LiClO4 solution. Moreover, the degree of the chemical interaction of Li+ ions with the sulfonate functional groups has been demonstrated by several methods such as FTIR and RAMAN studies. The FTIR peak shift at 1140 cm-1 and Raman shifts at 1155 cm-1 corresponding to the S=O symmetric stretching represent the interaction taking place between Li+ ions with the membrane. AFM studies has revealed that hydrophilic and hydrophobic domains have been well defined for acid and salt (Li+ and Na+) form of the membranes
Figure 1. BPSH Copolymer membrane
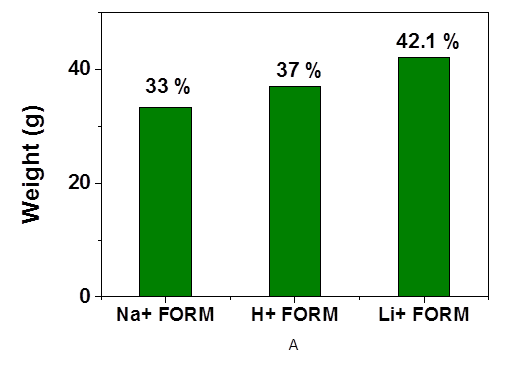
Recent Publications
[1] Su L, Darling R M, Gallager K G, Xie W, Thelen J L, Badel A F, Barton J L, Cheng K J, Balsara N P, Moore J S, Brushett F R (2016) An Investigation of the Ionic Conductivity and Species Crossover of Lithiated Nafion 117 in Nonaqueous Electrolytes. Journal of The Electrochemical Society 163(1): A5253-A5262
[2] Huang Q, Yang J, Ng C B, Jia C, Wang Q (2016) A redox flow lithium battery based on the redox targeting reactions between LiFePO4 and iodide. Energy & Environmental Science 9: 917-921
References
- Semiz L, Abdullayeva N, Sankir M (2018) Nanoporous Pt and Ru catalysts by chemical dealloying of Pt-Al and Ru-Al alloys for ultrafast hydrogen generation. Journal of Alloys and Compounds 744: 110-115.
- Serin R B, Abdullayeva N, Sankir M (2017) Dealloyed Ruthenium Film Catalysts for Hydrogen Generation from Chemical Hydrides. Materials 10(7): 738
- Abdullayeva N, Sankir M (2017) Influence of Electrical and Ionic Conductivities of Organic Electronic Ion Pump on Acetylcholine Exchange Performance. Materials 10(6): 586
- Akay T E, Abdullayeva N, Sankir M, Sankir N D (2016) On-Board Hydrogen Powered Proton Exchange Membrane Fuel Cells. ECS Transactions 75(14):511-513
- Sankir M, Semiz L, Sankir N D (2015) Catalyst free Hydrogen generation from directly disulfonated poly(arylene ether sulfone) copolymer Membranes. Journal of Membrane Science 496
