
Arun M. Umarji
Indian Institute of Science, India
Title: Aluminium quenched Brownmillerite type SrCoO2.5 for oxygen enrichment
Biography
Biography: Arun M. Umarji
Abstract
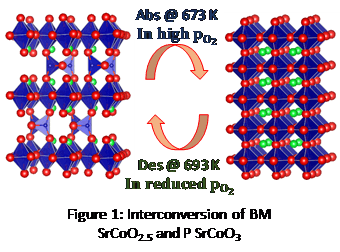
Ternary oxides are known for many applications like solid oxide fuel cells, catalysis, gas sensing etc [1]. These oxides can be oxygen non-stoichiometric if transition metals with variable oxidation states are present. This can be regarded as a functionality if the extent of oxygen vacancies inside the lattice is very high. They can transport oxygen through the lattice when a partial pressure gradient of oxygen is applied. This is normally exploited in case of Oxygen separation membranes [1]. Perovskite oxides (general formula ABO3) are promising candidates as they can exist in a variety of oxygen non-stoichiometric forms with varying temperature and oxygen partial pressure. SrCoO3 (Perovskite, Pm m) is one of such material which reversibly transforms to SrCoO2.5 (Brownmillerite, Ima2) phase at 350°C [2]. Here in we report the application of Brownmillerite SrCoO2.5 for oxygen enrichment.
Brownmillerite (BM) SrCoOx phase has been stabilized to date only by liquid nitrogen quenching [3]. We report a novel and cost-effective method of quenching for the synthesis of the BM SrCoOx. T. Dasgupta et al. [4]reported a quenching method using Al foil pads to control oxygen stoichiometry of REBaCo2O5+d. Herein, we extend this method to stabilize the BM phase of SrCoO2.5. The solution combustion synthesized powder was calcined and sintered at 1223 K in pellet form. This pellet was quenched to 473 K using Al foil pads to stabilize the intact ceramic with Brownmillerite phase. A simple home-built volumetric setup has been fabricated for studying the Oxygen storage property of the material [5]. The sample was pre-treated with a higher partial pressure of oxygen at 673 K to form the oxygen-rich perovskite phase and this phase was heated at lower pressure to study the desorption characteristics. The pressure change observed when sample releases oxygen is used to find the oxygen storage capacity. Desorption characteristics of the sample treated at varying oxygen partial pressure have been studied. The results indicate 14.72 litres/kg of oxygen can be stored in the sample at STP.
Image
Recent Publications
[1] J. Sunarso, S.S. Hashim, N. Zhu, W. Zhou, Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review, Prog. Energy Combust. Sci. 61 (2017) 57–77. doi:10.1016/j.pecs.2017.03.003.
[2] H. Jeen, W.S. Choi, M.D. Biegalski, C.M. Folkman, I.-C. Tung, D.D. Fong, J.W. Freeland, D. Shin, H. Ohta, M.F. Chisholm, H.N. Lee, Reversible redox reactions in an epitaxially stabilized SrCoOx oxygen sponge., Nat. Mater. 12 (2013) 1057–63. doi:10.1038/nmat3736.
References:
[1] Y. Takeda, R. Kanno, T. Takada, O. Yamamoto, M. Takano, Y. Bando, Phase Relation and Oxygen-non-stoichiometry of Perovskite- like Compound SrCoOx (2.29 < x < 2.80), Z. Anorg. Allg. Chem. 541 (1986) 259–270.
[2] T. Dasgupta, S. Sumithra, Arun M. Umarji, A novel method to control oxygen stoichiometry and thermoelectric properties in (RE)BaCo2O5+δ, Bull. Mater. Sci. 31 (2008) 859–862.
[3] Aswathy M. Narayanan, Rajasekar P., Arun M. Umarji, Stabilization of Brownmillerite type SrCoO2.5 by a Costeffective Quenching method for Oxygen Scavenging Applications, Submitt. to Ind. Eng. Chem. Res. (2018).
